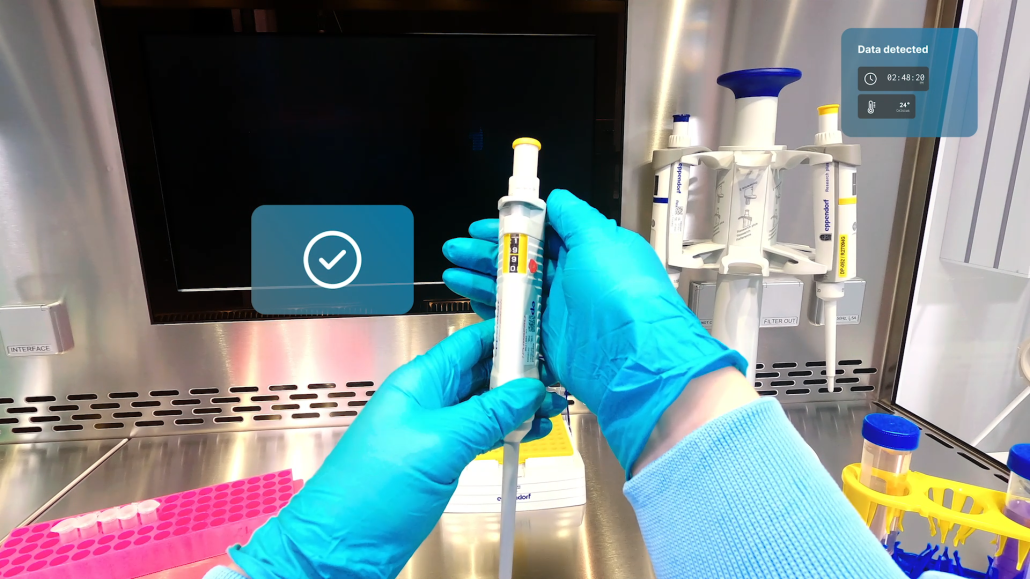
The process of establishing a new radiopharmaceutical at a new production site.
Need
- Knowledge has to be transferred to the operators via training in repeated face-to-face interactions
- Information has to be conveyed into the quality system of the receiving site via exchange of documents, which must be kept up to date over the whole lifecycle of the product
- Transfer activities must be well documented to proof they were successful based on predefined criteria
- Even after formal completion of the process support is often required from the sending site for troubleshooting or re-trainings


Opportunity
- Turn your paper-based documents into cloud-based instructions enriched with AR guidance
- Share this knowledge and information with new sites by a few clicks in form of ready-to-use SOPs and executable production and testing records
- Let the system guide new operators through the process with step-by-step instructions using hands-free smart glasses supported by intelligent computer vision models
- Support remotely as needed with AR-featured live audio/video calls
Solution
- Transfer your knowledge and documents once into DOCS
- Share the whole set with new production sites in a controlled way in seconds
- Train operators independently with ASSYST workflows
- Automatically document transfer/ training activities
- Shorten transfers and save up to 80% of resources at the sending site